What is in the Solar System?The Sun
The Sun is our nearest star. The Sun contains approximately 98% of the mass of the solar system and provides the gravitational force required to keep the planets orbiting around it. Solar energy is created deep within the core by nuclear fusion. Every second 700 million tons of hydrogen are converted into Helium. And the energy produced by this conversion is what gives energy to almost everything that lives on the Earth.
The Planets
In order of distance from the Sun, Mercury, Venus, Earth, Mars, Jupiter, Saturn, Uranus and Neptune. The inner planets (Mercury, Venus, Earth and Mars) are formed from solid material while the outer planets (Jupiter, Saturn, Uranus and Neptune) are formed from gas and liquid.

The Discovery of Neptune
(Since antiquity, the planets Mercury, Venus, Mars, Saturn, Jupiter).
Neptune could have been discovered a lot earlier than it was, Galieo had seen it
Newtons law of gravity allowed the solar system to be modelled with great accuracy, however observations of Uranus showed that it did not behave entirely as predicted. The only planet to be discovered was Uranus in 1781 by Music Teacher, William Herschel who believed it to be a comet and named it Georgium Sidus (George's Star) in honour of King George III.
Accurate data for the orbits of the other planets was available but the data for Uranus did not fit with the calculated results. The anomalous were caused by another large object and Neptune was discovered by calculating where a planet would have to be to produce the anomalous behavior of Uranus. This was the first planet to be discovered by mathematics.
The first accurate predictions of Uranus' motion were published in 1792. Within a few years, it was obvious that there was something wrong with the motion of the planet, as it did not follow the predictions. Attempts to calculate improved tables using the latest mathematical techniques were unable to fit all the observations, ancient and modern, to a single orbit. A commonly accepted suspicion during the next few years was that Newton's Law of Gravity, although accurate out to the orbit of Saturn, might not work in the same way at greater distances; but by the late 1830's it seemed at least equally likely that there was an unknown body lying beyond the orbit of Uranus.
Neptune was discovered on September 23, 1846 by the astronomer Johann Galle and his student Heinrich d'Arrest, after only thirty minutes of searching the sky, within a degree of the position predicted by the Urbain Le Verrier. This was the high-water mark of Newtonian physics: to be able, given the laws of physics and the peculiar motion of one object, to reach out into the depths of space and uncover a previously hidden object and caused an even greater sensation than the discovery of Uranus
There were a number of observations of Neptune prior to its discovery as a planet, but once again, mostly at times when it was near a stationary point, and hence nearly motionless relative to the stellar background. In fact, the first observations were made by Galileo, he even noted that the object which we now know as Neptune seemed to have moved relative to the nearest star, but failed to follow up his observations, and hence lost the opportunity to discover Neptune 168 years before Herschel discovered Uranus.
A planet had been discovered by mathematics, was it possible that other planets might be found in this way? A search at both ends of the solar system followed. Close to Mercury, the precession of the line of apsides (the imaginary line that runs along the major axis of the orbit). Unfortunately, even in the 1840's, it was obvious that the calculated rate of precession for the line of apsides was too small however, it might have conceivebly been caused by a smaller planet than Mercury that orbited on an orbit closer than Mercury. The planet was christened Vulcan (hence the name for the hot planet that Spock supposedly came from in the Star Trek series). The planet does not exist even though it has been observed on two occassions. The precession an effect of special and general relativity. It is ironic, given the early suposition that Uranus that the orbits errors were due to a flaw in Newtonian physics and the subsequent discovery that they were due to Neptune, that in the case of Mercury, the situation was exactly opposite. Initial speculations centred on a unknown planet and the solution involving a failure of Newtonian physics.
Around some of the planets there are moons which orbit the planet.
Dwarf Planets
At the time of writting, the IAU has officially reconises three Dwarf planets however there are other objects which may be designated as dwarf planets.
Ceres - located in the main asteroid belt was discovered in Jan 1st, 1801 by Giuseppe Piazzi. It is the smallest of the dwarf planets.
Pluto is not now considered to be a planet but a dwarf planet.
2003 UB313 formerly known as Xena is now officially named Eris - the largest known dwarf planet
Sedna - the coldest most distant place known in the solar system; possibly the first object in the long-hypothesized Oort cloud.
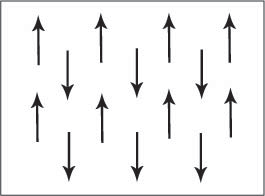 alignment of electron spins
alignment of electron spins
 a repulsive force will be felt. Similarly if a South pole is brought close to the South pole of another magnet, the two magnets will repel each other.
a repulsive force will be felt. Similarly if a South pole is brought close to the South pole of another magnet, the two magnets will repel each other.




